Happy Birthday CQAF! We turned 10 in 2020!!
生日快乐CQAF!2020年我们十岁啦!
With one decade down and more decades to go, we celebrated our 10th birthday modestly on Sep. 18th with more than 200 members from global and local pharmaceutical companies, clinical research organizations who attended the meeting on-line or in person.
十年荏苒,走向未来,我们于9月18日与来自线上线下的全球和本地制药公司、研究合同组织的200多位会员共同庆祝了CQAF十岁生日。
Unlike our past annual meeting, we held the meeting in two separate location, in the office of MSD in Beijing and Shanghai. This is in keeping with the safety measures to prevent the spread of COVID-19.
与往次年会不同,我们在默沙东北京和上海的两个办公室召开了会议。这符合防止新冠病毒扩散的安全措施。
Nonetheless, this did not dampen our excitement in this COVID era. COVID-19 has certainly enforced a new norm. It sped up digital transformation and without a doubt, Year 2020 will leave an indelible mark in the world’s history and the history of clinical research as well as CQAF.
但是,这并没有削减我们在这个特殊时期的参会热情。新冠病毒显然已经催生了新的标准。它加速了数字化转型,毫无疑问,2020年将在世界历史,临床研究以及CQAF的历史上留下不可磨灭的印记。
Our meeting was kicked off by Hannah Chen, the force behind CQAF. Her opening speech was full of vigor and she never failed to awe the audience with her professional life stories littered with much humor and wisdom.
CQAF Hannah Chen开启了我们的会议。她的开幕词充满活力,她的职业生涯故事充满幽默和智慧,从未使听众失望。
To commemorate our 10th year anniversary, a few invited members (Ellyne Setiawan, Hua(Maggie) Zhao, Yanping(Tracy) Liu , Yuanyuan Shi) from the audience shared their personal experience of how their lives become entwined with CQAF.
为了纪念我们成立10周年,观众中的一些受邀成员(Ellyne Setiawan, 赵华, 刘艳萍, 施媛媛)分享了他们与CQAF交织在一起的个人经历。
The CQAF working group also prepared a special video which took all of us down memory lane; starting from the birth of CQAF to where we are today. The inspiring message from first core team members (Hannah Chen, Zuoxiang Yu and Helen Li ) who shared a common belief and commitment, to promote GxP quality standards for the healthcare in China, had paved the way to establish the platform for quality professionals to share knowledge and best practices in the GxP area. The video also featured the congratulatory messages CQAF received from all over the world, including industry leaders e.g (Tongyan Wang and Carol Zhu from DIA, Deb Driscoll from MSD, Matt Jones from RQA) who had crossed path(s) with CQAF.
CQAF工作组还准备了一个特殊的视频,记录CQAF从诞生到今天,使我们所有人都记忆犹新。来自第一批核心团队成员(陈华,虞左向和李庆红)的鼓舞人心的寄语,他们有着共同的信念和承诺,以促进中国医疗保健的GxP质量标准,为建立高质量的专业人员共享知识和最佳实践的平台铺平了道路。 该视频还展示了CQAF从世界各地收到的祝贺信息,其中包括与CQAF有合作的各行业的领导者们(例如,DIA 的王彤琰和朱立红, 默沙东的Deb Driscoll, RQA的Matt Jones)。
After a cake-cutting ceremony both in Beijing and Shanghai, the session on Remote Audit & Inspection commenced. Our guest speaker, Jolie Weintraub, shared with us her rich experience on remote auditing and remote inspection. The insights also left the audience with food for thoughts on how we can incorporate her insight and experience into our current &/or future work relevant to remote audit and remote inspection. Our next speaker was Sean Xu who presented his hands-on experience on remote investigator site audit; encompassing the audit challenges faced during COVID-19 as well as remote auditing capability. His topic drew much interest from the audience. Guiqin Yu wrapped up the session on Remote Audit & Inspection. She wowed the audience with a comprehensive view on remote audit from definition, background, how to identify remote audit candidates as well as useful tips and hints for effective remote audits.
在北京和上海切蛋糕仪式后,远程稽查与检查经验分享部分开始了。我们的演讲嘉宾Jolie Weintraub与我们分享了她在远程稽查和远程检查方面的丰富经验。同时还使我们对于如何将她的见解和经验纳入我们与远程稽查和远程检查相关的当前和/或将来的工作中进行了思考。我们的下一位发言者是Sean Xu,他介绍了他在远程稽查研究中心方面的实践经验;包括新冠病毒流行期间面临的稽查挑战以及远程稽查可能性。他的话题引起了观众的极大兴趣。于桂琴总结了关于远程稽查和检查的部分。她让听众从定义,背景,如何识别远程稽查候选人以及有效的远程稽查提示等方面,对远程稽查有了全面的了解。
Our afternoon session focused on the topic of Quality Strategies to Maximize Business Values. Ellyne Setiawan (Boehringer Ingelheim (China) Investment Co., Ltd) , Linda Lu (Brii Bioscience) , and Yuyan Zhu (Tigermed) shared a glimpse of the Quality Strategies from their company. The purpose was to give the audience a flavor of how different organizations adopted QMS to fit the organization. We also organized an interactive panel session to engage with the meeting participants on burning questions and challenges they faced on different topics within the scope of QMS.
下午会议我们重点讨论了最大化企业价值的质量策略这一主题。Ellyne Setiawan(勃林格英格翰), 陆峰(腾盛博药)以及朱余艳(泰格)分享了他们公司的质量策略。目的是让听众了解不同组织如何采用QMS使其适合组织发展。我们还组织了一个互动式讨论,与会者就QMS在不同方面所面临的严峻问题和挑战展开了互动讨论。
There’s a famous saying that time flies when everyone is having a good time. This is true indeed; as before we realized it, the time had arrived to wrap up our meeting. It had been a fruitful day with lots of good exchanges and sharing. All slides presentation and Q&A will be published in the CQAF website and wechat platform.
我们总是说:时光总在美好瞬间中流逝。的确如此。像我们之前意识到的那样,现在该结束会议了。这是富有成果的一天,有很多有意义的交流和分享。所有幻灯片演示和问答将在CQAF网站和微信平台上发布。

CQAF 2020 Ten Years Celebration Meeting, Shanghai

CQAF 2020 Ten Years Celebration Meeting, Beijing
-----------------------------------------------------------------------------------------------------------
10 Years Celebration and Opening
Presentation by Amy Jiang / Hannah Chen/ Yifeng Shen
At the beginning of the annual meeting, we watched a video about CQAF’s history and the message from core team. You may refer to the link for more details.
年会一开始,会员们一起观看了CQAF发展历程的一个短片,聆听了核心团队对CQAF的寄语。您可以点击 链接 获取更多信息
Hannah shared her experience as quality person. Independ/critical thinking; respect profession and courage are the top three characteristics for QA/quality professionals.
陈华分享了她作为质量人的经验,在诸多必备的职业能力中,独立思考/批判性思维,敬畏专业和勇气是需要具备的重要的职业特质。
Dr. Shen emphasized that
沈老师强调:
l The discussion of conforming to the regulatory requirements and adapt to the rapid expansion of clinical trial in CHINA.
讨论符合法规要求并适应中国临床试验的快速发展
l QUALITY is the key word for local pharmaceutical companies go abroad.
质量是本土药企走向世界的核心
l Diversified members and cross disciplinary skills ASSURANCE us with encyclopedic knowledge and solutions.
成员多元化和跨学科技能确保我们拥有多元文化和解决方案。
l In the FORUM, every member’s voices can be heard and respected. Not only complaints, but also in-depth analysis and strategic solutions.
l 在CQAF, 我们尊重每位会员,这不仅是抱怨的声音,还有深入的分析和战略解决方案。
Topic 1: Remote Audit & Inspection
Presentation by Jolie Weitraub
Experience from two case studies, i.e. Remote Site Audit, and Study Level Audit, were shared with the participants. The remote audits and health authority inspections with regards to the rationale/background, further considerations, challenges, approaches/insights were discussed followed by interactive Q&A. The best practices for remote audit consideration including communication, preparation, process clarity, consideration of remote audit options, development of processes, and how to cope with remote inspections challenges were deeply shared and discussed.
与参与者分享了两个远程稽查案例的经验,即远程研究中心稽查和整个试验层面的稽查。讨论了远程稽查和药监部门检查相关的背景、进一步考虑因素、挑战、方法/洞见,并进行了互动式问答。深入交流并讨论了考虑远程稽查的最佳实践经验,包括沟通、准备、选择的考虑、流程,以及如何应对远程检查的挑战。
Topic 2: Remote investigator site audit and Remote Auditing Considerations
Presentation by Guiqin Yu, Sean Xu
Remote investigator site auditing capability focusing on the strategy and decision tree for the traditional, remote and centralized investigator site audits were shared. Tools for centralized audits such as examples of worksheets were introduced. In addition, the remote audit definition, background, evolvement, selection, tips and hints for Effective Remote Audits, and remote audit/inspection survey results were summarized taking the following into account including the technical, risk & criticality considerations, benefit and challenges in remote setting. The practical aspects and future outlooks were envisaged. This session was ended with hot Q&As.
从策略和决策树的角度分享了远程研究中心稽查, 包括传统稽查、远程稽查和中心化(centralized)稽查的可能性,并介绍了中心化稽查工具,例如工作表示例。结合远程稽查/检查的问卷调查结果,回顾了远程稽查的定义、背景、发展历程、如何选择,并分享了达成有效远程稽查的建议和注意事项,以及对远程稽查需要考虑的因素,包括技术工具的使用、风险和关键性数据、远程稽查的收益和挑战, 同时讨论了实操层面和未来前景的设想。本次会议在热烈的问答环节中进行。
Topic 3: Create and adopt agile & fit-for-purpose quality strategies for different types of organizations, in order to maximize your business values
Presentation by Ellyne Setiawan, Linda Hu, Julia Zhu
Panel: Hannah Chen, Cathy Liu, Liping Zhou, Ellyne Setiawan, Linda Hu, Julia Zhu
Speakers from 3 different types of pharma-organizations had shared their experience in establishment and deployment of clinical quality strategies. Due to the varieties of company characteristics, development stages and business goals, the agile and fit-for-purpose quality strategies were designed and adopted in order to ensure the quality of clinical development activities and provide the assistants in maximizing the business values. For example, in a global pharmaceutical company where the clinical QMS (cQMS) had been fully established, the focusing area in the quality strategy may be translated into how the established cQMS could better facilitate employee’s work in the organization. In the direction of the quality strategy, more investments may be spent on quality culture cultivation, SOP simplification and learning & knowledge management. In contrast, for a newly formed biotech company, in order to speed up the development of company’s valuable assets, a more flexible and risk-based quality strategy may be applied to create a valid and practical cQMS in a short period of time. Another example was given by a local company who expanded their business by acquiring external companies all over the world. It became challenging in the establishment of a group-wide cQMS which adherences to an unified quality standard within the organization. An end-to-end QMS integration plan, including concept agreement, role mapping, process integration, training program and audit activity, played a crucial role during the oversea subsidiaries’ integration.
来自3种不同类型的制药企业的演讲者分享了他们在建立和部署临床质量策略方面的经验。由于公司特征,发展阶段和业务目标的多样性,因此设计并采用了灵活,适合目的的质量策略,以确保临床开发活动的质量并为最大化企业价值提供帮助。例如,在一家已经完全建立了临床质量管理体系(cQMS)的跨国制药公司中,质量策略的重点可以转化为如何使cQMS更好地促进员工在组织中的工作。在质量战略的方向上,可能会在质量文化的培养,简化SOP和学习与知识管理方面投入更多的资金。相比之下,对于一家新成立的生物技术公司而言,为了加快公司宝贵资产的开发速度,可能会采用一种更灵活,基于风险的质量策略来在短时间内创建有效且实用的cQMS。另一个例子是一家本地公司,该公司通过收购世界各地的外部公司来扩展业务。建立整个组织范围内的统一质量标准的cQMS变得具有挑战性。一项完整的端到端cQMS整合计划,包括统一概念,角色映射,流程整合,培训计划和稽查活动,都在海外子公司的整合中起着至关重要的作用。
The presentations were followed by a panel discussion consisting of the speakers and other invited experts from global pharmaceutical companies and QA consulting firm. TransCelerate cQMS elements and foundations were actively discussed, including resource, roles & responsibilities, processes, partnering, knowledge management and management commitment to quality. At the end, all the distinguished speakers provided positive comments on the question “what will the future QA look like in the next 5-10 years”. So, what do you think?
在演讲之后,演讲者和来自全球制药公司和QA咨询公司的受邀专家们以小组讨论的形式讨论了TransCelerate cQMS的要素和基础,包括资源,角色和职责,流程,合作伙伴,知识管理以及对质量的管理承诺。最后,所有嘉宾都对“未来5-10年内的质量检查将是什么样子”这一问题给予了积极评价。所以,小伙伴们,你怎么看?
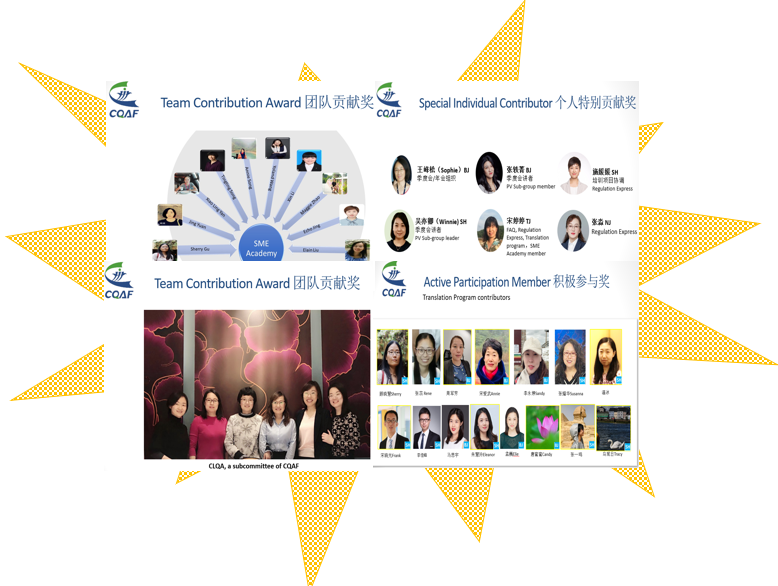
Annual Award
CQAF members’ engagement and contribution significantly contributes to the continuous development and success of CQAF. We took the precious time to express our gratitude and reward those individuals or groups who proactively participated in CQAF projects, initiatives and quarterly meetings.
CQAF会员的参与和付出为CQAF的持续发展和成功做出了巨大贡献。我们特抽出宝贵的时间对那些积极参加CQAF项目、倡议活动和季度会议的个人或团体表示感谢和奖励。
We sincerely appreciated your active participation to the 10-year’s celebration and 2020 Annual meeting. Look forward to your continuous support and engagement to future events.
衷心感谢您积极参与10周年庆典和2020年年会。期待您继续支持和参与以后的活动。

-----------------------------------------------------------------------------------------------------------------------------------------------
If you have any ideas or proposals for how we can do better, please go to the link or QR code below to submit your thoughts.
如果您对我们如何做得更好有任何想法或建议,请转到下面的链接或QR码提交您的想法。

关于上述的分享PPT,请登陆会员账户,点击下方第二页。
关于如何注册和登陆会员账户,请参见 CQAF Website User Guide

 沪公网安备 31011202013414号
沪公网安备 31011202013414号